The resources below have been provided to help narrow your search to specific, targeted drug information. Information is available for both consumers and healthcare professionals on over 24,000 prescription and over the counter medicines available primarily in the USA.
Title 21: Food and Drugs List of Subjects revised as of October 1, 2020. 21 CFR Part 1General enforcement regulations. Cosmetics Drugs Exports Food labeling Imports Labeling Reporting and recordkeeping requirements 21 CFR Part 2General administrative rulings and decisions. Administrative practice and procedure Cosmetics Drugs Foods 21 CFR Part 3Product jurisdiction. Administrative practice. The drug is subject to an open drug efficacy study implementation (DESI) program proceeding, health care professionals rely on the drug to treat serious medical conditions when there is no FDA. A drug already approved by the Licensing Authority mentioned in Rule 21 for certain claims, which is now proposed to be marketed with modified or new claims, namely, indications, dosage, dosage form (including sustained release dosage form) and route of administration. Fixed dose combination of two or more drugs, individually approved earlier.
Browse Alphabetically
Browse Drugs by Category
Popular Drug Searches
Consumer Drug Sources
Cerner Multum Consumer Drug Information
Multum leaflets provide basic consumer drug information, such as drug descriptions and interactions, details of possible side effects and the effects of missed doses and overdosing, as well as instructions for use. The leaflets are available in English and Spanish.
IBM Watson Micromedex Consumer Information (Advanced)
IBM Watson Micromedex Advanced Consumer Information provides comprehensive consumer information pertaining to a wide variety of drugs, such as a list of commonly used brand names, drug descriptions, warnings and precautions, and detailed information on the proper use of each drug.
Consumer Drug Information
Consumer Drug Information provides detailed information on drug indications, contraindications and interactions as well as notes on safety and instructions for use.
Professional Drug Sources
AHFS DI Monographs

AHFS DI from the American Society of Health-System Pharmacists' (ASHP) is the most comprehensive source of unbiased and authoritative drug information available to health professionals today. A wholly independent staff of drug information pharmacists and other professional editorial and analytical staff thoroughly research AHFS DI content. Authors incorporate clinical research findings, therapeutic guidelines, and Food and Drug Administration (FDA) approved labeling to ensure that monographs include an evidence-based foundation for safe and effective drug therapy.

FDA Professional Drug Information
The Professional Drug Information database is a repository of drug information sourced directly from the FDA. It includes detailed notes on the clinical pharmacology of a wide variety of drugs.
Professional Patient Advice
Drugs.com provides A-Z Patient Advice for the professional.
Natural Product Sources
Natural Product Information (Consumer)

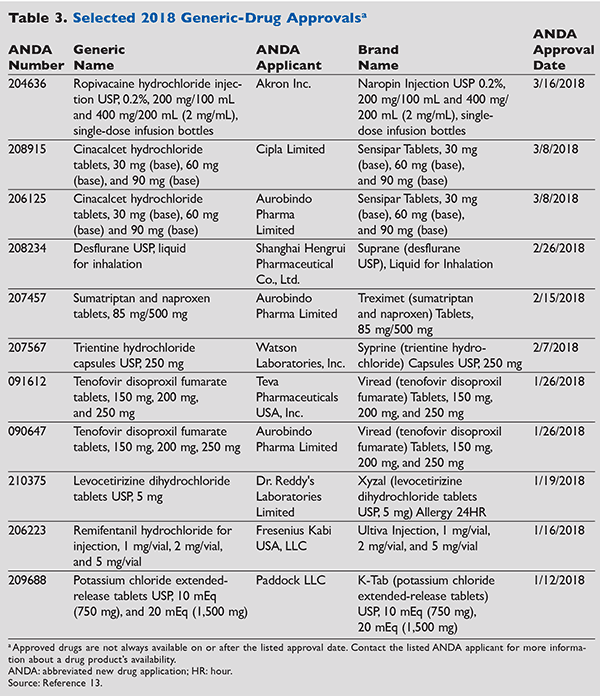
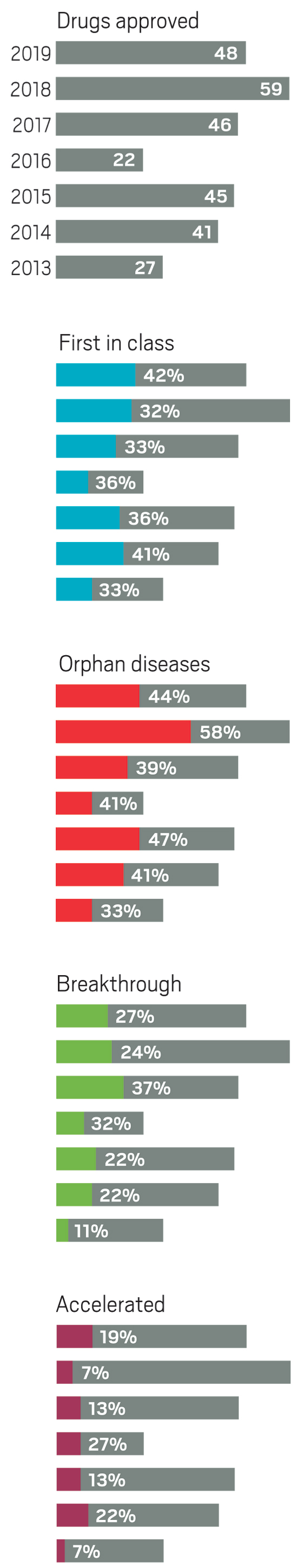
Subject: New Drug Names Types
The Natural Product Information (Consumer) database is a comprehensive source of information on traditional and/or conventional uses of natural products. A basic overview of each product is provided (including dosages, possible drug interactions, side effects and contraindications) along with safety and/or efficacy ratings.
Natural Product Information (Professional)
Subject: New Drug Names A-z
The Natural Product Information (Professional) database is a comprehensive source of information on traditional and/or conventional uses of natural products. A basic overview of each product is provided (including dosages, possible drug interactions, side effects and contraindications) along with safety and/or efficacy ratings.
